The Purple Book isn’t a novel you read for fun-it’s the U.S. Food and Drug Administration’s official database for biological medicines, including biosimilars and interchangeable products. If you’re a pharmacist, doctor, or patient trying to understand what can be swapped for what, this is the single most important resource you need to know. Unlike a printed book, it’s online, searchable, and updated in real time. And as of 2026, it’s the only place you’ll find clear, federal-level answers about which biological drugs are interchangeable and which aren’t.
What Exactly Is the Purple Book?
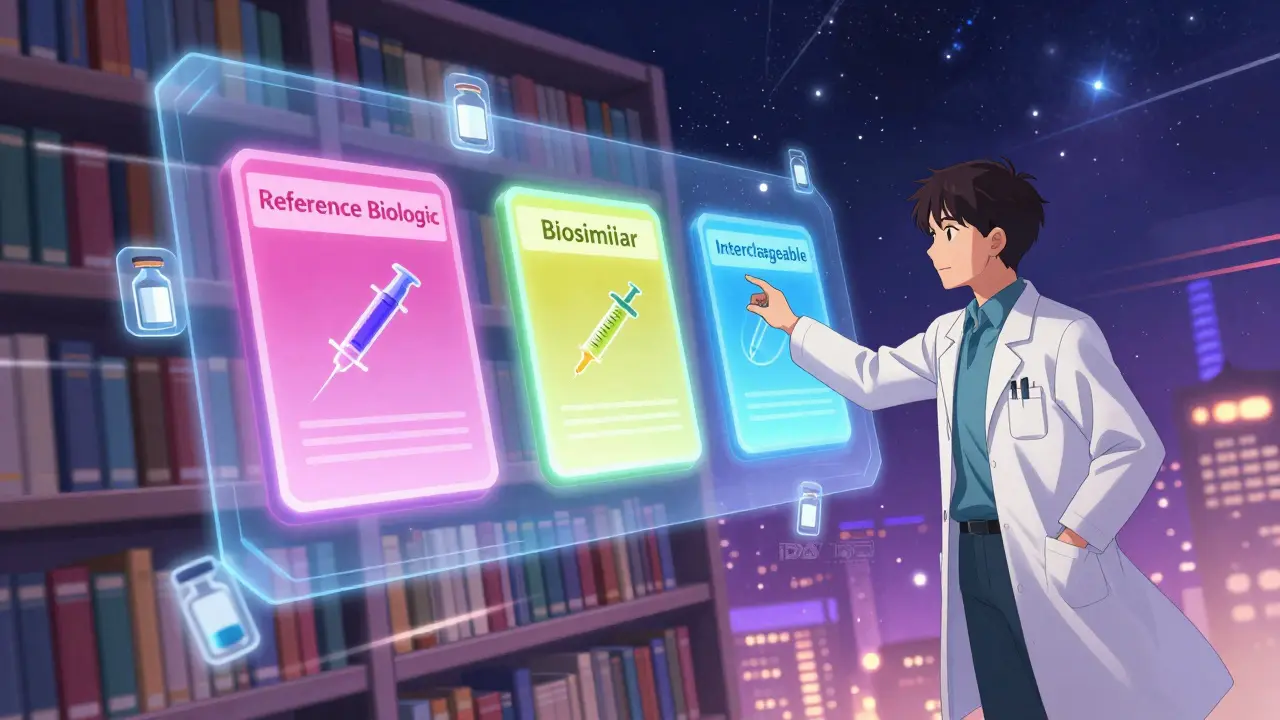
The Purple Book is a searchable database maintained by the FDA that lists every approved biological product in the U.S. That includes the original brand-name biologics, their biosimilar copies, and the few that have been officially labeled as interchangeable. It was created under the Biologics Price Competition and Innovation Act (BPCIA) of 2010, but it wasn’t until 2020 that the FDA merged two separate lists-one for drugs under CDER and one for biologics under CBER-into one unified system. Before that, finding information was messy. Now, it’s all in one place.Each product in the Purple Book gets a color-coded card. Matching colors mean products are related-like a reference biologic and its biosimilar. You’ll see the brand name, the generic name, the date it was approved, and whether it’s a 351(a) reference product, a 351(k) biosimilar, or a 351(k) interchangeable. Icons show delivery methods too-autoinjectors, prefilled syringes, vials. It’s designed so pharmacists can quickly scan and know what’s available.
Biosimilars vs. Interchangeable: The Key Difference
This is where people get confused. All interchangeable products are biosimilars. But not all biosimilars are interchangeable. Think of it like this: a biosimilar is a very close copy of the original biologic. It’s not identical-no two biological molecules are-but it’s so similar that there are no meaningful differences in safety or effectiveness.An interchangeable product goes further. To earn that label, the FDA requires proof that switching back and forth between the biosimilar and the original doesn’t increase risk or reduce effectiveness. That means if a patient uses the reference drug for three months, then switches to the biosimilar for three months, then switches back-there’s no drop in results or spike in side effects. This isn’t just about being similar. It’s about being predictable in repeated use.
The FDA’s definition is clear: an interchangeable product must show that the risk of alternating between it and the reference product is no greater than using the reference product alone. That’s a higher bar than biosimilarity. And it’s why only a handful of biosimilars have made it to interchangeable status.
Why Does Interchangeability Matter?
Interchangeability isn’t just a technical label-it affects what happens at the pharmacy counter. In most states, pharmacists can swap an interchangeable biosimilar for the prescribed brand-name drug without asking the doctor. That’s not true for regular biosimilars. For those, the prescriber has to specifically write “do not substitute.”As of 2024, 47 states and Puerto Rico allow pharmacists to make this substitution without prior approval from the prescriber. But rules vary. Some states require the pharmacist to notify the doctor. Others require patient consent. A few still don’t allow substitution at all. That means even if a product is federally approved as interchangeable, whether you get it instead of the brand depends on where you live.
And here’s something important: the FDA stresses that interchangeable doesn’t mean better. It doesn’t mean safer. It doesn’t mean more effective. It just means you can switch between it and the original without added risk. The science behind both biosimilars and interchangeable products is solid. The difference is in the level of evidence needed for approval.

What’s in the Purple Book Right Now?
As of late 2023, only seven biosimilars had received interchangeable status from the FDA. That number is growing, but slowly. The approved interchangeable products include:- Two insulin products (used for diabetes)
- Three treatments for inflammatory conditions like rheumatoid arthritis and Crohn’s disease
- Two therapies for eye conditions affecting the retina
These are all high-cost, complex biologics. Their interchangeable versions are expected to bring down prices over time. But adoption isn’t automatic. Even with FDA approval, manufacturers still need to convince payers, hospitals, and pharmacies to switch. Many are still hesitant, partly because of state-level confusion and partly because of lingering misconceptions.
The Purple Book also lists every reference biologic and every biosimilar that’s been approved-even if it’s not interchangeable. So if you’re looking for a biosimilar version of Humira, Enbrel, or Herceptin, you’ll find it. The database shows which biosimilars have been approved, when, and against which reference product.
How to Use the Purple Book
It’s free, public, and easy to use. Go to the FDA’s website, search for a brand name like “Humira,” and you’ll see the original product and every biosimilar or interchangeable version that’s been approved to match it. The results are grouped under the reference product. That’s intentional. It helps you see the whole family of related products at once.Use the filters to narrow by product type-insulin, autoimmune, oncology-or by status: biosimilar, interchangeable, or reference. You can also check the date of approval and whether the product has exclusivity protection. That’s important because some reference products have a 12-year exclusivity period before biosimilars can even apply for approval.
Pharmacists use it daily to verify substitution rules. Patients can use it to check if their prescribed drug has a cheaper alternative. Providers use it to understand what’s available when writing prescriptions. It’s not a marketing tool. It’s a factual, regulatory resource.

What’s Not in the Purple Book
Don’t expect pricing info. You won’t find out how much a biosimilar costs compared to the brand. That’s up to insurers and pharmacies. You won’t find patient reviews or side effect reports either. Those come from other FDA systems like MedWatch.And don’t confuse “unbranded biologic” with “interchangeable.” An unbranded biologic is a version that’s not marketed under a brand name, but it hasn’t gone through the interchangeability process. The FDA considers it equivalent, but it’s not legally interchangeable under pharmacy substitution laws.
What’s Next for Biosimilars?
More applications for interchangeable status are coming. Companies are investing in the extra clinical studies needed to prove switching safety. The FDA has released draft guidance on labeling to make sure these products are clearly marked. That’s a big step-it means pharmacists and patients won’t be confused about what they’re getting.But progress is slow. The science is there. The regulatory path is clear. What’s holding things back is complexity: state laws, payer policies, provider education, and patient trust. The Purple Book gives you the facts. But changing how the system works takes time.
For now, if you want to know what’s approved, what’s interchangeable, and what can be substituted, the Purple Book is your starting point. It’s not perfect. But it’s the most accurate, up-to-date, and official source you’ve got.
Is the Purple Book the same as the Orange Book?
No. The Orange Book lists approved generic drugs-small-molecule medicines like ibuprofen or metformin. The Purple Book lists biological products-larger, complex molecules made from living cells, like insulin or antibodies. They’re two different systems for two different types of medicines.
Can a pharmacist substitute a biosimilar without a doctor’s permission?
Only if the biosimilar has been designated as interchangeable by the FDA and your state allows substitution. Regular biosimilars require a prescriber to write "dispense as written" or "no substitution." Interchangeable products can be swapped automatically in most states, but rules vary. Always check your state’s pharmacy laws.
Are interchangeable biosimilars safer than regular biosimilars?
No. The FDA says interchangeable biosimilars are not safer or more effective than other biosimilars. The only difference is that they’ve passed extra studies proving you can switch between them and the original drug multiple times without increased risk. Both types are equally safe and effective when used as directed.
Why are so few biosimilars interchangeable?
Because proving interchangeability requires additional clinical trials-specifically, studies that test switching back and forth between the biosimilar and the reference product. These studies are expensive and time-consuming. Many manufacturers choose to skip them and focus on getting biosimilar approval, which is enough to enter the market. Interchangeability is a bonus, not a requirement.
Where can I find the Purple Book?
Go to the FDA’s official website at fda.gov and search for "Purple Book." It’s a free, public database. No login required. You can search by brand name, generic name, or therapeutic category. It’s updated regularly as new products are approved.
Does the Purple Book include pricing information?
No. The Purple Book only shows regulatory status-approval date, reference product, interchangeability designation. It does not include prices, insurance coverage, or cost comparisons. Those details are managed by insurers, pharmacies, and pharmacy benefit managers.
Final Thoughts
The Purple Book is a quiet revolution in how we access biologic medicines. It brings transparency to a space that was once shrouded in confusion. For too long, patients and providers didn’t know which alternatives were legally allowed, which were scientifically sound, and which could be swapped without permission. Now, you can find the answers in minutes.It’s not a cure-all. State laws still create patchwork rules. Insurance companies still control access. But the Purple Book gives you the truth. And in a world full of marketing claims and misinformation, that’s powerful.





Shelby Price
February 3, 2026 AT 09:55Just checked the Purple Book for my dad’s insulin-found two interchangeable options I had no idea existed. Saved him like $300/month. This thing is a lifesaver.
❤️
Keith Harris
February 4, 2026 AT 23:44Oh please. The FDA’s Purple Book? More like the Purple Mirage. Half these ‘interchangeable’ labels are just corporate lobbying dressed up as science. You think they’d let a biosimilar swap if it didn’t cut their profits? Please. The real difference? The brand-name companies still own the pharmacy shelves. This ‘transparency’ is a marketing gimmick wrapped in regulatory jargon.
And don’t even get me started on how states are still playing jurisdictional tug-of-war. It’s a circus. A very expensive, very boring circus.
Samuel Bradway
February 6, 2026 AT 08:55I work in a rural clinic and this changed everything. Before, we’d spend hours calling reps or digging through PDFs. Now? Five minutes in the Purple Book, we know exactly what’s swappable, what’s not, and who’s got the data to back it up.
Even our patients are asking smarter questions now. That’s huge. Thanks for highlighting this-it’s not flashy, but it’s the backbone of real change.
Prajwal Manjunath Shanthappa
February 6, 2026 AT 15:03One must, however, exercise considerable discernment when engaging with the FDA’s Purple Book-lest one fall prey to the insidious myth of ‘interchangeability’ as a panacea. The regulatory architecture, while ostensibly transparent, remains profoundly opaque to the layperson. The very notion that a biosimilar, however statistically proximate, could be deemed ‘interchangeable’ without longitudinal, real-world pharmacovigilance data is, frankly, preposterous. One wonders whether the FDA has been co-opted by the biotech lobby-or worse, is simply overreaching under the guise of ‘efficiency.’
Ed Mackey
February 8, 2026 AT 14:18so i just found out my insurance only covers the biosimilar if it's marked 'interchangeable' in the purple book? i had no idea. thanks for the heads up. i'm gonna check it right now. also, i think i misspelled 'interchangeable' in my last search. oops.
Katherine Urbahn
February 10, 2026 AT 07:15It is imperative to underscore that the FDA’s designation of ‘interchangeability’ does not, under any circumstances, imply superior efficacy, safety, or clinical superiority. To conflate interchangeability with enhanced therapeutic value is not merely incorrect-it is a dangerous misrepresentation of regulatory science. The distinction between biosimilar and interchangeable is not semantic-it is evidentiary, methodological, and legally codified. Any healthcare provider who fails to comprehend this distinction is unfit to prescribe.
Alex LaVey
February 10, 2026 AT 14:05Hey everyone-just wanted to say how awesome it is that we finally have a single, clear source for this stuff. I’ve seen so many patients stressed out because they didn’t know if their new insulin was ‘the same’ or if they’d get in trouble for switching. The Purple Book doesn’t solve everything, but it gives people a place to start with confidence.
And hey, if you’re a pharmacist or a doc reading this-pass this link along to your colleagues. We’re all in this together.
caroline hernandez
February 11, 2026 AT 19:03From a clinical pharmacy standpoint, the Purple Book is the only authoritative source for 351(k) product lineage. The color-coding system is a brilliant UX innovation-pharmacists can triage substitution workflows at a glance. But the real bottleneck? PBM formularies and prior authorization protocols that still treat biosimilars as ‘second-tier.’ Until payers align with FDA labeling, we’re just moving paper, not access.
Also: don’t confuse ‘unbranded’ with ‘interchangeable.’ That’s a common error in EHR templating. Flag it when you see it.
Joseph Cooksey
February 13, 2026 AT 17:54Let’s be brutally honest here: the entire biosimilar ecosystem is a Rube Goldberg machine of regulatory loopholes, corporate profit motives, and state-level chaos. The FDA, bless its bureaucratic heart, has done its part-created a clean, searchable database that actually works. But then you’ve got pharmacy chains that still refuse to stock biosimilars because ‘patients won’t accept them,’ and insurers who won’t reimburse unless you jump through 17 hoops, and doctors who still write ‘do not substitute’ out of sheer habit, not science.
Meanwhile, patients are paying $2,000 for a drug that has a $400 interchangeable version sitting right there in the Purple Book. And we’re still talking about ‘education’ like it’s a soft problem. It’s not. It’s a moral failure wrapped in a spreadsheet.
Justin Fauth
February 14, 2026 AT 06:05USA is the only country that lets Big Pharma charge $100K for a biologic. Now they slap a ‘interchangeable’ sticker on a copy and call it ‘progress’? Please. This isn’t innovation-it’s a delay tactic. The Purple Book? Yeah, it’s useful. But if we really wanted to lower costs, we’d stop letting patent trolls hold patients hostage for 12 years. We’re not fixing the system-we’re just giving it a new color.
Amit Jain
February 14, 2026 AT 11:04Simple: Purple Book = official list. Find your drug. See if it has a cheaper version that can be swapped. Use it. No need to overthink.