Every year, over 90% of prescriptions filled in the U.S. are for generic medications. Yet, if you walk into a pharmacy and see a pill that looks different from the one you’ve been taking, you might hesitate - even if your doctor says it’s the same. Why? Because perception isn’t always shaped by science. It’s shaped by color, shape, cost, and trust.
What Exactly Is a Generic Drug?
A generic drug isn’t a cheaper copy. It’s the exact same medicine, with the same active ingredient, strength, dosage form, and route of administration as the brand-name version. The FDA requires it to be bioequivalent - meaning it delivers the same amount of drug into your bloodstream at the same rate as the brand. That’s not a guess. It’s proven through strict tests measuring how your body absorbs the medicine.
The FDA’s Orange Book sets the standard: the ratio of absorption between the generic and brand must fall between 80% and 125% for both total exposure (AUC) and peak concentration (Cmax). That’s a tight window. If a generic doesn’t meet this, it doesn’t get approved. And here’s the kicker: the same factories that make brand-name drugs often make generics too. The FDA inspects over 1,500 generic drug facilities every year - the same standards apply.
Doctors Know Generics Work - So Why Don’t They Always Prescribe Them?
Major medical groups like the American College of Physicians have been clear since 2016: prescribe generics when possible. Their reasoning? Patients are 6% more likely to stick with their medication if it’s cheaper. And better adherence means fewer hospital visits, fewer complications, and lower overall costs.
But here’s the disconnect. While 90% of prescriptions are filled with generics, only 72% of new prescriptions are written as generics. That means doctors are still starting patients on brand-name drugs more often than they should be.
Why? It’s not about ignorance. A 2016 study of 151 physicians found no link between a doctor’s belief in generic cost savings and their prescribing habits. So what’s driving the choice?
- habit - Many doctors prescribe what they were taught in medical school, and that’s often the brand.
- patient pressure - 41% of physicians say patients sometimes demand the brand-name version, even when they know it costs 10 times more.
- fear of change - Some doctors worry that switching a stable patient to a generic might cause problems, even though evidence shows it rarely does.
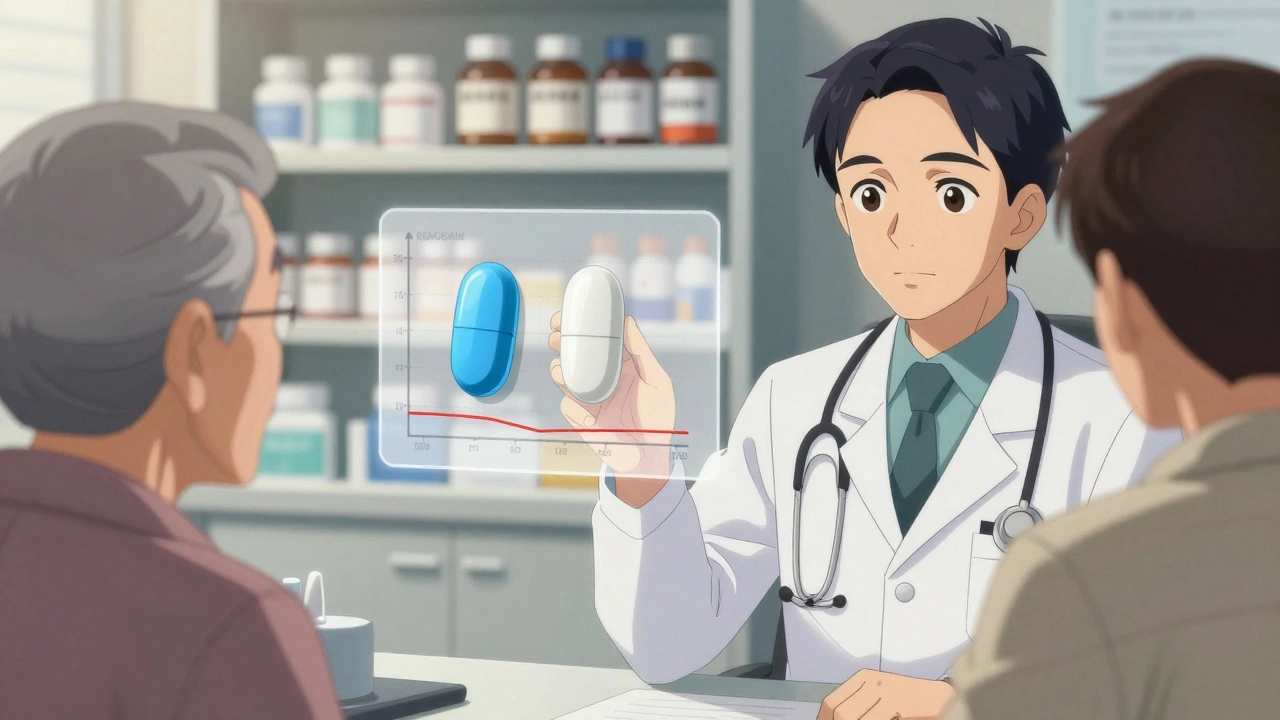
One internist in Melbourne told me, “I had a patient refuse lisinopril because the generic was white and oval, not blue and round. He thought the color change meant it was weaker. I showed him the FDA data. He still didn’t trust it.”
Patients Don’t Trust Generics - And It’s Not Just About Price
Price is a big reason people choose generics. But the real barrier is perception. Patients see a different pill - different color, different shape, different packaging - and assume it’s different medicine. The FDA calls this the “look-alike, sound-alike” problem. Since 2018, their program has cut patient confusion by 37% by standardizing labeling and improving communication.
Still, doubts linger. In FDA surveys, patients say they worry about:
- “Will it work as well?”
- “Are the inactive ingredients safe?”
- “Why would the company make a cheaper version if it’s not just as good?”
These aren’t irrational fears. They’re human reactions. Your brain associates familiarity with safety. When your blood pressure pill suddenly changes from blue to white, your mind goes to: “Did something go wrong?”
And it’s not just patients. Even doctors admit they feel uneasy with certain drugs. The FDA lists 15 medications with a narrow therapeutic index - like warfarin, levothyroxine, and phenytoin - where tiny differences in blood levels can cause real harm. For these, some doctors prefer to stick with the brand, even though generics are approved and tested.
Cost Isn’t Just a Number - It’s a Lifeline
Generics cost 80-85% less than brand-name drugs. That’s not marketing. That’s fact. The Congressional Budget Office estimates that if doctors started prescribing generics for every new prescription, Medicare Part D could save $17.3 billion a year.
But money isn’t just about budgets. It’s about access. A 2021 Kaiser Family Foundation study found Medicare beneficiaries who took generics had 12.7% higher adherence rates than those on brand-name drugs. That’s not a small gap. That’s thousands of people staying on their meds, avoiding ER visits, and living longer.
One Canadian study tracked 136,000 seniors on blood pressure meds. When generics became available, hospitalizations went up - but researchers suspect it wasn’t because the drugs were worse. It was because people stopped taking them. They couldn’t afford the brand anymore, so they skipped doses. That’s the real danger: not the generic drug. It’s the cost of not taking it.
Where the System Still Falls Short
Here’s the truth: the system works - but only if everyone understands how.
Pharmacists can legally substitute generics in 49 states. But in some places, the doctor has to write “dispense as written” on the prescription to block the switch. Many patients don’t know that. Many doctors don’t know that.
And education gaps are real. In Saudi Arabia, 96% of doctors said they understood the value of generics - but only 16% said they used them in all cases. In Greece, half of doctors rated generic quality as high or very high - yet only 25% prescribed them regularly.
Why? Because knowledge doesn’t always change behavior. You can know something’s true and still feel uneasy about it. That’s psychology, not science.
But there’s progress. Since 2015, the number of internal medicine residency programs teaching generic prescribing has jumped from 29% to 68%. Doctors who took FDA-sponsored training saw a 23% increase in generic prescribing within six months.
What You Can Do - As a Patient or a Caregiver
You don’t need to be a doctor to make smart choices. Here’s how to take control:
- Ask - “Is there a generic version of this?” Don’t assume there isn’t. Many brand-name drugs have generics you didn’t know about.
- Check - Use tools like GoodRx or your pharmacy’s price list. You might be paying $350 for a brand when the generic is $4.
- Talk - If your pill looks different, ask the pharmacist. They can explain the change and confirm it’s the same medicine.
- Track - If you feel different after switching, write it down. Not because the generic is bad - but because your body might need time to adjust, or you might be reacting to a new filler.
- Advocate - If your doctor won’t prescribe a generic, ask why. Is it based on evidence? Or habit?
One woman in her 70s told me she’d been on the same brand of cholesterol pill for 12 years. When her insurance switched her to the generic, she refused. Her daughter showed her the FDA’s bioequivalence data. She started taking it. Three months later, her cholesterol dropped - same as before. She said, “I thought I was being loyal to the brand. Turns out, I was just paying extra.”
The Future Is Generic - But Trust Takes Time
By 2030, over 85% of prescriptions will be for generics. Biosimilars - the next generation of generic biologics - are starting to roll out. The FDA expects them to make up 15% of biologic prescriptions by 2027.
But technology won’t fix mistrust. Education will. Communication will. Trust will.
Doctors aren’t resisting generics because they’re ignorant. Patients aren’t rejecting them because they’re irrational. We’re all responding to decades of branding, marketing, and fear.
The solution isn’t more data. It’s more conversation. A pharmacist explaining why the pill changed. A doctor saying, “This is the same medicine. I’ve prescribed it to hundreds of patients. It works.” A patient asking, “Can you show me the proof?”
Because in the end, medicine isn’t about pills. It’s about trust. And trust is built one conversation at a time.





Chase Brittingham
December 5, 2025 AT 01:09Been on a generic blood pressure med for five years now. Same exact results, no side effects, and I save $280 a month. My grandma switched too after her pharmacist showed her the FDA paperwork. She said she felt weird at first because the pill looked different, but now she won’t go back to the brand. Sometimes it’s just about getting used to the new look.
Joe Lam
December 7, 2025 AT 00:07Let’s be real - if generics were truly identical, why do pharmaceutical companies spend billions marketing brand names and designing pill aesthetics? The FDA’s bioequivalence standards are a joke. 80-125% absorption window? That’s a 45% variance. You wouldn’t let a pilot fly with a 45% margin of error, so why trust your life to a pill that might be half as potent? This isn’t science - it’s corporate propaganda dressed up as regulation.
Rachel Bonaparte
December 7, 2025 AT 21:40Joe, you’re missing the point. The 80-125% range isn’t arbitrary - it’s statistically validated across thousands of clinical trials. The FDA doesn’t approve generics based on wishful thinking. And before you go full conspiracy, ask yourself: why do the same factories that make Lipitor also make atorvastatin? Why do hospitals run double-blind studies using generics and get identical outcomes? It’s not a conspiracy. It’s manufacturing efficiency. The real conspiracy is how Big Pharma tricks people into thinking color and shape = efficacy.
Chad Handy
December 9, 2025 AT 03:45I used to be the guy who refused generics until my wife got diagnosed with hypothyroidism. We switched her from Synthroid to levothyroxine because insurance wouldn’t cover the brand anymore. First month, she had brain fog, fatigue, panic attacks. We thought the generic was garbage. We went back to the brand - and she felt fine. Then we found out the pharmacy had switched her to a different generic manufacturer mid-month. We switched back to the original generic - same as before. Turns out, the first batch she got was from a different supplier, and the fillers were triggering her anxiety. Not the active ingredient. The binders. So now I’m not anti-generic - I’m pro-consistency. If your doctor switches you, ask which manufacturer. Stick with one. Don’t let the pharmacy shuffle you around like a deck of cards.
And yes, I’ve spent hours on the phone with pharmacies arguing about this. I’ve sent emails to the FDA. I’ve printed out the FDA’s list of approved manufacturers. I’ve become the generic drug guy. Not because I love generics - because I learned the hard way that not all generics are created equal, even if the active ingredient is the same.
Doctors don’t tell you this. Pharmacists don’t tell you this. But if you’re on a narrow-therapeutic-index drug - levothyroxine, warfarin, phenytoin - you need to know your manufacturer. Write it down. Demand it. Your life depends on consistency, not just equivalence.
I’ve seen people die because they got switched from one generic to another without warning. It’s not paranoia. It’s documentation. And if your doctor rolls their eyes when you ask for the manufacturer code, find a new doctor.
Generics are fine. But the system is broken. And we’re the ones paying the price.
Scott van Haastrecht
December 9, 2025 AT 08:00Chad, you’re not a hero - you’re a pawn. You think you’re saving money, but you’re just enabling the system that lets drug companies charge $1,200 for a pill that costs $0.50 to make. The real villain isn’t the pharmacy or the doctor - it’s the patent system that lets companies monopolize drugs for 20 years, then sell the same molecule for decades as a ‘brand.’ And now you’re patting yourself on the back for taking a $4 pill instead of a $350 one? Congrats. You’re still playing their game. The only real solution is single-payer, price caps, and ending pharmaceutical patents entirely. Until then, you’re just a well-meaning sucker.
Jenny Rogers
December 10, 2025 AT 03:44It is, indeed, a profound moral failing of contemporary medical culture that the patient’s psychological attachment to pharmaceutical aesthetics is permitted to supersede empirical evidence. The FDA’s bioequivalence thresholds, while statistically robust, are insufficiently communicated to the layperson, thereby permitting a pernicious epistemological gap to flourish - one wherein the placebo effect of brand recognition is erroneously conflated with therapeutic efficacy. One cannot, in good conscience, advocate for cost containment while simultaneously permitting irrational affective responses to pill morphology to dictate clinical outcomes. The solution is not merely education, but institutional re-education - a paradigmatic shift in how medicine is perceived as a discipline grounded in rationality, not branding.
Michael Feldstein
December 10, 2025 AT 19:21My dad’s on warfarin. We switched him to generic a year ago. No issues. But I get why people are scared. I used to work in a pharmacy - I’ve seen patients cry because their pill changed color. It’s not just about the drug. It’s about control. When you’re sick, your body feels foreign. The pill is the one thing you can hold onto. Change that, and you feel like you’ve lost your grip. So yeah, I get it. But here’s what helps: pharmacists who take five minutes to explain it. Doctors who say, ‘I’ve prescribed this to 300 people. No one’s had a problem.’ It’s not the pill. It’s the conversation.
zac grant
December 11, 2025 AT 11:55For those concerned about bioequivalence ranges: the 80-125% CI is derived from log-transformed AUC and Cmax data using a 90% confidence interval per FDA guidance. It’s not a 45% window - it’s a statistically validated equivalence zone calibrated to minimize clinical variability. The FDA requires intra-subject variability to be <30% for narrow-therapeutic-index drugs. So if your Cmax is 115% of brand, you’re still within 95% of the expected pharmacokinetic profile. Bottom line: generics are safe. The fear is psychosocial, not pharmacological.
jagdish kumar
December 12, 2025 AT 08:03Same medicine. Different color. People cry. Sad.
Augusta Barlow
December 13, 2025 AT 09:47Did you know the FDA allows generic manufacturers to use talc, corn starch, and dye that aren’t in the brand version? And those ‘inactive ingredients’? They’re not inert. Some cause allergic reactions. Some interfere with absorption. The FDA doesn’t test for long-term effects of these fillers - only short-term bioequivalence. So yes, the pill might ‘work’ - but is it safe? Who’s to say what happens after ten years of daily talc exposure? I’ve seen studies linking food-grade dyes in pills to ADHD in children. And no one’s talking about it. Because the system doesn’t want you to know.
Bill Wolfe
December 14, 2025 AT 16:58Oh wow, another person who thinks the FDA is some saintly guardian of public health. 🤡 Let’s not forget: the same FDA that approved OxyContin now approves generics made in the same Chinese factories that got shut down for falsifying data. And you’re telling me I should trust a white oval pill from a facility that had 17 FDA warning letters? I’ve seen the inspection reports. They’re not fixing the problems - they’re just paying fines and moving on. The FDA isn’t protecting you. It’s protecting profits. Generics are cheaper because they’re made cheaper. That’s it. Don’t be fooled by the ‘same active ingredient’ fairy tale. Your body isn’t a lab rat.
Benjamin Sedler
December 16, 2025 AT 09:50Here’s the real problem nobody wants to admit: generics are the reason pharmacies can charge $4 for a 30-day supply. It’s not altruism - it’s leverage. Insurance companies force generics because they’re cheaper, not because they’re better. And once you’re on a generic, they lock you in. No choice. No options. You think you’re saving money? You’re just trapped in a system that treats your health like a commodity. The brand-name drug? It’s not the enemy. The system is. And we’re all just rats in a maze, arguing over which wheel spins faster.