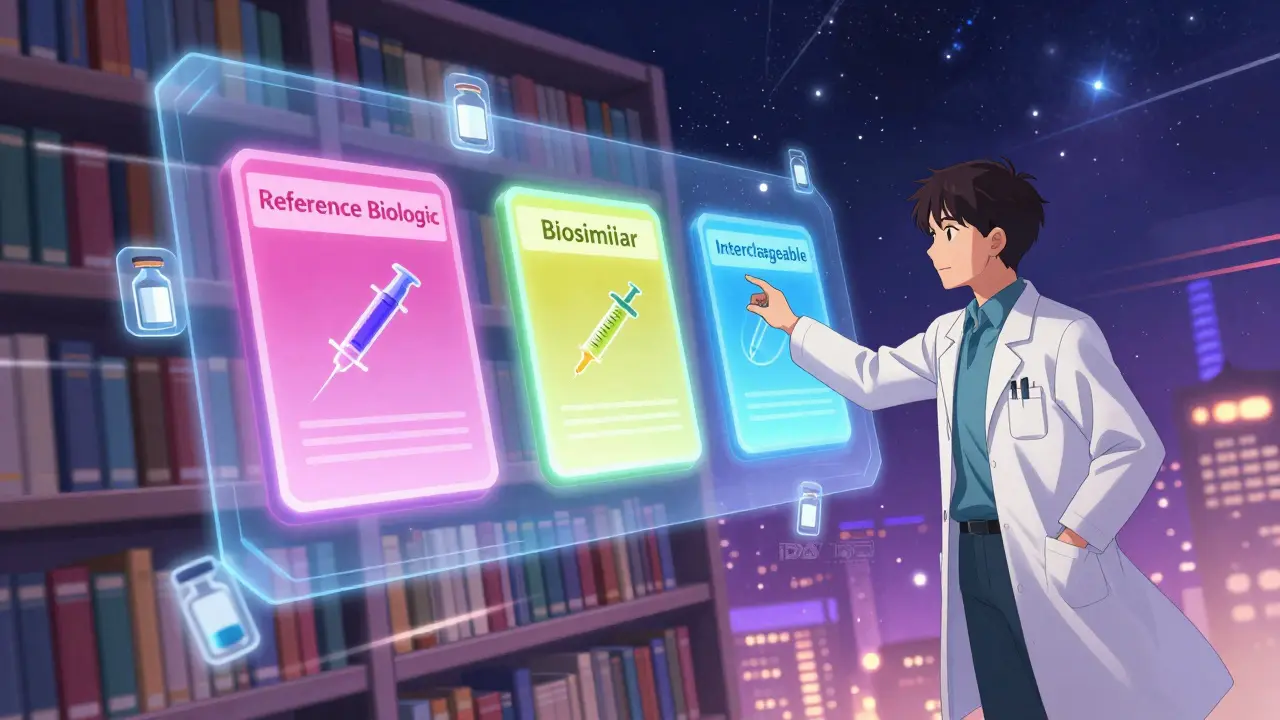
The FDA's Purple Book is the official database for biological products, biosimilars, and interchangeable drugs. Learn how it works, what interchangeability means, and why it matters for patients and pharmacists.
Read More
Pharmacists play a vital role in biosimilar adoption by counseling patients, navigating complex substitution laws, and ensuring safe, cost-effective care. Learn how they drive adoption and overcome barriers in biologic therapy.
Read More