Bone Health Marker Comparison Tool
Compare Key Bone Health Markers
This tool shows how laboratory values differ between healthy individuals and those with secondary hyperparathyroidism (SHPT).
Normal Values
Secondary Hyperparathyroidism (SHPT) Values
Key Differences in SHPT
- Elevated PTH: Often exceeds 300 pg/mL, indicating overactivity of parathyroid glands.
- High Phosphate: Levels rise above normal due to impaired kidney function.
- Low Vitamin D: Deficiency is common due to reduced kidney activation of vitamin D.
- Increased ALP: Reflects high bone turnover and ongoing bone remodeling.
- Altered Calcium: May be low-normal or slightly elevated due to PTH action.
Key Takeaways
- Secondary hyperparathyroidism (SHPT) is common in chronic kidney disease and drives high bone turnover.
- Elevated parathyroid hormone (PTH) disrupts calcium‑phosphate balance, leading to bone loss and increased fracture risk.
- Lab markers such as alkaline phosphatase, serum calcium, and phosphate help track bone damage.
- Treatment focuses on controlling PTH, correcting vitamin D deficiency, and using phosphate binders.
- Lifestyle measures - adequate protein, weight‑bearing exercise, and fall‑prevention - complement medical therapy.
What is Secondary Hyperparathyroidism?
Secondary Hyperparathyroidism is a hormonal disorder that arises when the parathyroid glands secrete excess parathyroid hormone (PTH) in response to low calcium or high phosphate levels, most often because of chronic kidney disease (CKD). The condition differs from primary hyperparathyroidism, where the glands are overactive on their own. In SHPT, the body’s attempt to normalize mineral balance ends up overstimulating bone resorption.
The cascade begins with reduced activation of vitamin D by diseased kidneys. Vitamin D a fat‑soluble vitamin essential for calcium absorption in the gut drops, leading to low serum calcium. The parathyroid glands react by releasing more Parathyroid hormone a peptide hormone that raises blood calcium by stimulating bone resorption, increasing renal calcium reabsorption, and activating vitamin D. Simultaneously, failing kidneys cannot excrete phosphate efficiently, so serum phosphate climbs, further stimulating PTH.
Secondary hyperparathyroidism therefore sits at the intersection of mineral metabolism and bone health, creating a high‑turnover bone disease often called renal osteodystrophy.
How SHPT Disrupts Bone Metabolism
Bone is a living tissue that constantly remodels through the coordinated actions of osteoclasts (which break down bone) and osteoblasts (which build new bone). PTH has a dual, dose‑dependent effect: short bursts of PTH stimulate bone formation, while sustained high levels trigger osteoclast‑mediated bone loss.
In SHPT, chronic elevation of PTH tips the balance toward resorption. This results in several recognizable changes:
- Increased osteoclastic activity - more bone matrix is removed, creating microscopic holes that weaken the structural framework.
- Reduced mineralization - newly formed bone may be poorly mineralized because calcium is constantly drawn into the bloodstream.
- Subperiosteal bone resorption - classic radiographic sign where bone is eaten away just under the periosteum, often seen on the phalanges.
- Development of osteitis fibrosa cystica - severe end‑stage lesion where fibrous tissue replaces bone, forming cyst‑like brown tumors.
Because the bone turnover is rapid, patients often experience a paradox of pain (due to microfractures) and a higher risk of overt fractures, especially in the hip, spine, and wrist.
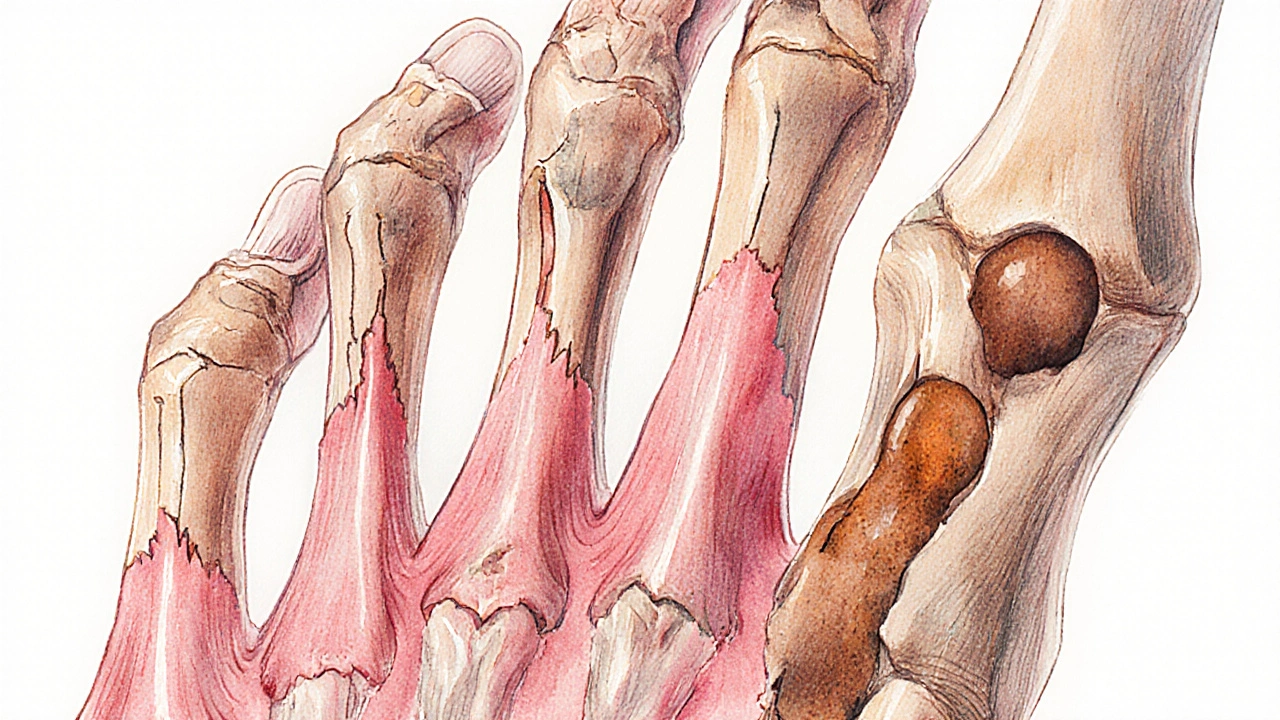
Clinical Indicators of Bone Damage
Physicians monitor several lab and imaging markers to gauge the impact of SHPT on the skeleton.
- Serum calcium the amount of calcium in the blood, usually kept between 8.5‑10.5 mg/dL - often low‑normal or slightly elevated due to PTH action.
- Serum phosphate phosphate concentration, which rises as kidney function declines - frequently above the normal range of 2.5‑4.5 mg/dL.
- Alkaline phosphatase (ALP) an enzyme released by active bone‑forming cells; elevated levels signal high bone turnover - can be two‑ to three‑fold higher than normal.
- Intact PTH assay measures the full, biologically active form of parathyroid hormone - values often exceed 300 pg/mL in advanced SHPT.
- Bone density (DXA) - dual‑energy X‑ray absorptiometry may show reduced BMD, but the rapid turnover can mask loss, so trabecular bone score (TBS) adds useful detail.
- Radiographs - subperiosteal erosions on hand films and “salt‑and‑pepper” skull appearance are classic signs.
Combining these clues helps differentiate SHPT‑related bone disease from other forms of osteoporosis.
Managing Bone Health in SHPT
Therapeutic goals are twofold: lower PTH to a target range (usually 150‑300 pg/mL) and correct mineral imbalances to stop bone loss.
- Phosphate binders - calcium‑based (calcium acetate) or non‑calcium (sevelamer) agents taken with meals reduce intestinal phosphate absorption, helping lower serum phosphate.
- Active vitamin D analogues - calcitriol or newer agents like paricalcitol boost calcium absorption and directly suppress PTH synthesis.
- Calcimimetics - cinacalcet increases the sensitivity of calcium‑sensing receptors on parathyroid cells, tricking them into thinking calcium levels are higher, thus reducing PTH secretion.
- Dialysis adequacy - optimizing dialysis dose improves phosphate clearance and can modestly lower PTH.
- Surgical parathyroidectomy - reserved for refractory cases where medical therapy fails; removes hyperplastic glands and can rapidly normalize PTH.
While these treatments address the hormonal driver, supporting the bone directly is also crucial.
Lifestyle & Nutrition for Stronger Bones
Patients can bolster their skeletal health with everyday choices:
- Protein intake - adequate protein (0.8‑1.0g/kg body weight) supports collagen matrix formation.
- Weight‑bearing exercise - activities like walking, stair climbing, or resistance bands stimulate osteoblast activity.
- Fall‑prevention strategies - clear home hazards, use slip‑resistant mats, and consider balance training.
- Calcium sources - dairy, fortified plant milks, leafy greens; aim for 1000‑1200mg/day unless contraindicated by high calcium‑based binder use.
- Vitamin D supplementation - if levels are below 30ng/mL, a typical regimen is 800‑2000IU daily, adjusted for CKD stage.
Regular monitoring every 3-6months allows clinicians to tweak therapy before irreversible bone damage occurs.

Comparison of Bone‑Health Markers: Normal vs. Secondary Hyperparathyroidism
| Marker | Normal Range | Typical SHPT Range |
|---|---|---|
| Serum Calcium (mg/dL) | 8.5-10.5 | 8.0-10.0 (low‑normal or slightly high) |
| Serum Phosphate (mg/dL) | 2.5-4.5 | 5.0-7.0 (elevated) |
| Intact PTH (pg/mL) | 10-65 | 150-800 (elevated) |
| Alkaline Phosphatase (U/L) | 30-120 | 200-600 (high‑turnover) |
| 25‑OH Vitamin D (ng/mL) | 30-100 | 15-30 (deficient) |
Next Steps & Troubleshooting
If you or a loved one have CKD and lab results show rising PTH, start a conversation with your nephrologist about the following checklist:
- Confirm CKD stage and dialysis regimen.
- Order a full mineral panel (Ca, Ph, PTH, ALP, 25‑OH D).
- Introduce a phosphate binder tailored to calcium load.
- Begin active vitamin D analogues if 25‑OH D is low.
- Consider calcimimetic therapy if PTH remains >300 pg/mL after 3 months.
- Schedule a bone density scan and, if available, a trabecular bone score.
- Re‑evaluate every 4-6 weeks; adjust meds based on trends, not single values.
Should PTH stay stubbornly high despite maximal medical therapy, discuss surgical options early-waiting too long can lock in irreversible bone loss.
Frequently Asked Questions
Why does kidney disease trigger secondary hyperparathyroidism?
Diseased kidneys lose the ability to convert inactive vitamin D to its active form and to excrete phosphate. Low active vitamin D reduces calcium absorption, while phosphate builds up. Both changes stimulate the parathyroid glands to release more PTH, leading to secondary hyperparathyroidism.
Can secondary hyperparathyroidism cause osteoporosis?
Yes. The chronic high‑PTH state accelerates bone turnover, thinning the trabecular network and raising fracture risk, which mimics or worsens osteoporosis.
What are the target PTH levels after treatment?
Guidelines suggest keeping intact PTH between 150 and 300 pg/mL for most CKD patients on dialysis. Values lower than 150 pg/mL may indicate over‑suppression, which can also harm bone.
Is surgery ever needed?
Parathyroidectomy is reserved for refractory SHPT when medications cannot lower PTH or when bone disease progresses despite optimal therapy. Success rates are high, but the procedure carries surgical risks.
How often should bone density be checked?
A DXA scan every 1‑2 years is reasonable for most CKD patients with SHPT, especially if they have a history of fractures or markedly elevated ALP.




Steven Elliott
October 9, 2025 AT 19:39Wow, because we totally needed another deep‑dive into how failing kidneys love to hijack our skeletons. It's not like doctors already have a mountain of guidelines to stare at. And sure, the PTH fireworks are just “fun” for the bone, right? I guess we should all clap for the parathyroid glands doing their “best” work.
Lawrence D. Law
October 16, 2025 AT 18:19It is incumbent upon the practitioner, therefore, to assay the relevant biochemical indices-serum calcium, phosphate, intact PTH, alkaline phosphatase, and 25‑OH vitamin D-with scrupulous exactitude; thereafter, therapeutic modalities may be instituted in a methodical fashion. Moreover, the utilisation of phosphate binders, active vitamin D analogues, and calcimimetics must be calibrated to the individual’s residual renal function. Finally, regular monitoring-every three to six months-ensures that the therapeutic window is neither exceeded nor neglected. Consequently, the risk of high‑turnover bone disease may be attenuated, preserving skeletal integrity.
Mary K
October 23, 2025 AT 16:59The dance between kidneys and bones is a saga that reads like a modern tragedy sprinkled with hope. When the kidneys falter, they stop converting vitamin D into its active form, leaving calcium craving the gut. Phosphate, meanwhile, piles up like unwanted guests at a party that never ends. The parathyroid glands, ever the over‑eager responders, crank out PTH as if they were auditioning for a fireworks show. That relentless surge of hormone tells bone to dissolve its own scaffold, freeing calcium into the blood. What results is a high‑turnover battlefield where osteoclasts devour and osteoblasts scramble to rebuild, only to be under‑nourished. Patients feel the paradox: bone pain from micro‑fractures alongside a frightening risk of full‑blown fractures in the hip, spine, and wrist. Laboratory markers become the story‑tellers: calcium teeters low‑normal, phosphate spikes, alkaline phosphatase soars, and PTH rockets past 300 pg/mL. Radiographs might reveal that eerie “salt‑and‑pepper” skull or subperiosteal erosions that look like tiny gulches on the hand. Yet, this is not a hopeless fate; therapy offers a toolkit as varied as a chef’s spice rack. Phosphate binders-calcium‑based or sevelamer-act like bouncers, stopping excess phosphate at the intestinal door. Active vitamin D analogues, such as calcitriol, bring back the sunshine vitamin to the gut, bolstering calcium absorption. Calcimimetics, the clever tricksters, make the parathyroid think there is plenty of calcium, dialing down PTH output. And when medicines falter, surgery steps in, excising the hyperactive glands and often delivering a rapid calm. All the while, patients should power up with weight‑bearing exercise, adequate protein, and fall‑prevention tactics to protect the skeleton from the storm.
Odin Zifer
October 30, 2025 AT 14:39They dont tell you that the pharma industry is pushing calcimimetics to keep us dependent its all a set up the labs are manipulated to make PTH look scary and the meds become mandatory
Marisa Leighton
November 6, 2025 AT 13:19Hey there! Don’t let those numbers scare you – you’ve got options and a team ready to help. First off, think of phosphate binders as tiny shields that stop extra phosphate from sneaking into your bloodstream. Pair that with active vitamin D, which acts like a sunshine boost for your gut, pulling more calcium in. If PTH is still partying hard, a calcimimetic can gently remind the glands to calm down. And remember, staying active with simple weight‑bearing moves – even a daily walk or light resistance band – tells your bones, “I’m still here, keep building!” Keep those blood tests on a regular schedule, and you’ll spot trends before they become problems. You’re not alone in this journey; lean on your nephrologist, dietitian, and support network. Together, you can steer those lab values back into a healthier range and protect your bones for the long haul. 🌟
Brennan Keeler
November 13, 2025 AT 11:59Look, Marisa’s pep talk is cute but the real-world data shows that many patients still end up on parathyroidectomy because the med regime fails. The guideline‑driven approach you described often ignores the fact that non‑calcium binders like seveLamer can cause GI upset, leading to non‑adherence. Plus, the cost of calcimimetics is prohibitive for most insurance plans – you cant just throw money at the problem. In short, the idealised protocol you sold is more theory than practice, and clinicians need to address those practical gaps now.
Shanmughasundhar Sengeni
November 20, 2025 AT 10:39One must concede that Brennan’s scathing appraisal, albeit laced with typographical exuberance, captures a fragmented glimpse of the therapeutic landscape. Yet, to reduce the discourse to mere cost‑benefit matrices neglects the nuanced interplay of patient‑centered care, pharmacodynamics, and the evolving evidence base. A more erudite deliberation would contemplate the stratification of CKD stages, individual mineral metabolism trajectories, and the emerging role of combination regimens. In essence, while the critique holds merit, it too falls short of the holistic synthesis required for true advancement.
ankush kumar
November 27, 2025 AT 09:19Brothers and sisters, let me take you on a little journey through the tangled web of secondary hyperparathyroidism and why it matters more than just a lab value on a paper. Imagine your kidneys as the diligent garbage collectors of the body, sweeping away excess phosphate and turning vitamin D into its active form, ready to soak up calcium from the meals you eat. When those collectors call in sick, phosphate starts hanging around like an unwelcome guest at a dinner party, and the vitamin D conversion line stalls, leaving calcium feeling left out and insecure. The parathyroid glands, ever the over‑achievers, interpret this crisis as a signal to crank up PTH production, essentially yelling “More calcium, please!” to the rest of the body. This hormonal shoutout leads to bone cells called osteoclasts stepping up their game, breaking down bone to release calcium into the bloodstream, a process that, while helpful in the short term, is nothing short of a slow‑burn demolition of your skeletal framework. Over months and years, this relentless cycle spits out a cascade of symptoms: bone pain that feels like a low‑grade ache, a higher propensity for fractures that can turn a simple fall into a life‑changing event, and a lingering sense of fatigue as the body’s resources are diverted to manage the mineral imbalance. Now, the good news is that we have a toolbox full of strategies to tackle this. Phosphate binders like calcium acetate or sevelamer act like traffic officers, stopping phosphate from being absorbed in the gut. Active vitamin D analogues, such as calcitriol, swoop in to help the intestines absorb more calcium, while calcimimetics like cinacalcet trick the parathyroid receptors into thinking there’s already plenty of calcium, dialing down PTH secretion. And for those stubborn cases where medication fails, surgical removal of the hyperplastic parathyroid tissue can bring rapid relief. The key, however, lies in vigilance: regular monitoring of calcium, phosphate, PTH, alkaline phosphatase, and vitamin D levels every few months allows clinicians to fine‑tune therapy before the bone damage becomes irreversible. So, whether you’re a patient navigating this maze or a caregiver seeking clarity, remember that knowledge, routine testing, and a proactive treatment plan are your best allies in preserving bone health amidst the challenges of chronic kidney disease.
Cameron White
December 4, 2025 AT 07:59That was a lot but basically, keep checking the labs and use binders, vitamin D, or meds to stop the bone loss.
Amélie Robillard
December 11, 2025 AT 06:39Sure, because nothing says "healthy bones" like a kidney‑induced hormone frenzy 🙃
Fae Wings
December 18, 2025 AT 05:19Oh the irony! While we joke, patients are battling micro‑fractures that feel like fireworks exploding in their joints. It's a stark reminder that behind the memes lie real pain and a fight for stability. Stay compassionate, folks! :)
Anupama Pasricha
December 25, 2025 AT 03:59Understanding the cascade from phosphate retention to elevated PTH really empowers patients to take charge of their health. By integrating diet modifications, consistent dialysis, and personalized medication plans, we can mitigate bone turnover and improve quality of life. It’s essential to foster a collaborative environment where clinicians listen to patient concerns and adjust therapy accordingly.
Bryce Charette
January 1, 2026 AT 02:39Exactly, Anupama. Regular labs and open communication keep everything on track; plus, a quick reminder to double‑check those calcium readings for any typos before making decisions.
Christina Burkhardt
January 8, 2026 AT 01:19When it comes to bone health in CKD, the synergy between proper mineral management and lifestyle factors can’t be overstated. Simple measures like daily walking, balanced protein intake, and fall‑prevention strategies act as a foundation upon which medical therapies build. By staying proactive, patients can maintain stronger bones and reduce fracture risk.
liam martin
January 14, 2026 AT 23:59In the grand theater of the body, the skeleton plays the quiet hero, bearing the weight of existence while the kidneys whisper chaos; balance, therefore, is the silent script that writes health.