Myeloma Follow-Up Schedule Calculator
Personalized Follow-Up Schedule
Enter your current treatment phase to see recommended follow-up frequency for multiple myeloma.
Living with Multiple Myeloma a cancer of plasma cells that builds up in the bone marrow, leading to bone lesions, anemia, and a weakened immune system means staying on top of your health every day. One of the biggest game‑changers isn’t a new drug or a breakthrough therapy - it’s the habit of showing up for regular check‑ups. Those appointments let doctors spot subtle changes before they turn into serious complications, and they give patients a clearer picture of where their disease stands.
Key Takeaways
- Consistent monitoring catches relapse or progression early, improving survival rates.
- Blood work, imaging, and occasional bone‑marrow tests form the core follow‑up package.
- The NCCN Guidelines recommend a personalized schedule based on treatment phase.
- Patients who actively track symptoms report better quality of life.
- Avoiding missed appointments is often the single most effective way to stay ahead of the disease.
Why Regular Check-Ups Matter
When you’re in remission, it can feel like the battle is over. In reality, multiple myeloma is a chronic condition that can linger at low levels for years. Regular check‑ups act like a weather‑forecast for your disease - they let you see if a storm is brewing. Studies from the International Myeloma Working Group show that patients who adhere to a structured follow‑up plan have a 15‑20% lower risk of skeletal‑related events and a measurable boost in overall survival.
Beyond the hard numbers, there’s a psychological benefit. Knowing exactly where you stand reduces anxiety and helps you make informed choices about work, travel, and family life.
Core Components of a Follow‑Up Visit
Most oncologists schedule a mix of laboratory tests, imaging, and occasional invasive procedures. Here’s what you can expect:
- Blood work: Complete blood count (CBC), serum calcium, creatinine, and serum free‑light‑chain (FLC) assays.
- Serum protein electrophoresis (SPEP) and immunofixation (IFE), which measure the M‑protein that signals disease activity.
- Imaging: Low‑dose whole‑body CT, MRI, or PET/CT to spot new bone lesions.
- Bone‑marrow biopsy (when needed) to directly assess plasma‑cell infiltration.
Each test has a purpose, and the combination paints a full picture of disease burden.
When to Schedule Each Test
Frequency isn’t one‑size‑fits‑all. The NCCN Guidelines a set of evidence‑based recommendations for cancer care, updated annually by expert panels suggest the following baseline schedule:
| Phase | Blood Tests | Imaging | Bone‑Marrow Biopsy |
|---|---|---|---|
| Induction therapy | Every 2‑4 weeks | Baseline only | Usually not needed |
| Post‑ASCT (first 12 months) | Monthly | Every 3‑6 months | At 6‑month mark if labs rise |
| Maintenance (year 2‑5) | Every 2‑3 months | Every 6‑12 months | Only if suspicion of relapse |
| Long‑term survivorship | Every 3‑6 months | Annually or as symptoms dictate | Rarely |
These intervals are adjusted based on individual risk factors - for example, patients with high‑risk cytogenetics or a history of rapid progression may need tighter monitoring.
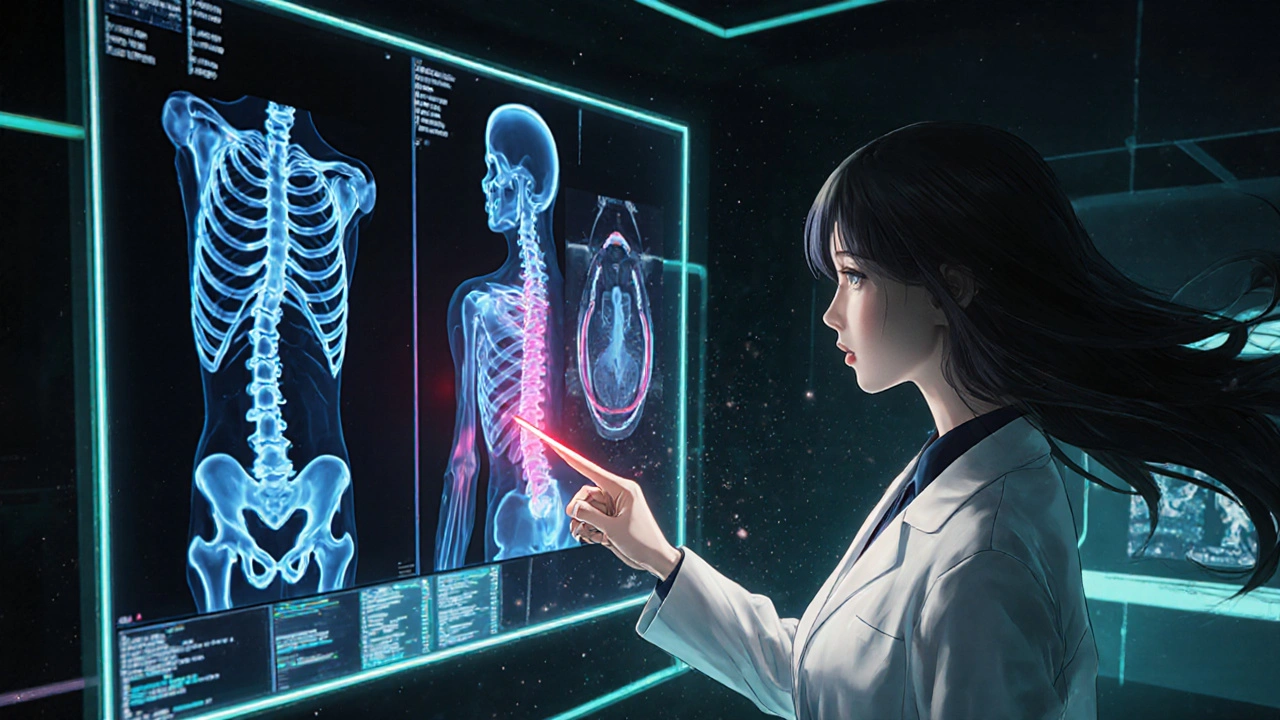
Understanding the Key Tests
Serum Protein Electrophoresis (SPEP) a lab technique that separates proteins in the blood to detect abnormal M‑protein spikes is the cornerstone of disease tracking. A rise of just 0.5 g/dL can signal early relapse, prompting a change in therapy before the patient feels any symptoms.
Bone‑Marrow Biopsy a procedure where a small sample of marrow is extracted, allowing direct counting of plasma cells offers the most definitive evidence of progression, especially when blood markers are ambiguous. Though invasive, it’s usually reserved for cases where imaging or SPEP results are inconclusive.
Imaging modalities each have strengths. MRI magnetic resonance imaging that excels at visualizing spinal and pelvic bone lesions can detect lesions hidden from X‑rays. PET/CT combines metabolic activity mapping with detailed anatomy, useful for spotting extramedullary disease is ideal when you suspect disease outside the skeleton.
Impact of Early Detection on Outcomes
When a relapse is caught early, physicians can restart therapy at a lower disease burden, often achieving a second remission that’s deeper and longer lasting. Data from the Myeloma IX trial indicate that patients who began second‑line treatment within 30 days of biochemical progression had a median overall survival of 68 months versus 53 months for those who delayed.
Early intervention also lowers the risk of skeletal‑related events (fractures, spinal cord compression). By treating bone disease sooner with bisphosphonates or denosumab, patients preserve mobility and independence.
Supporting Your Appointments: Practical Tips
- Keep a health journal: Record pain levels, fatigue, and any new symptoms. Bring it to every visit.
- Set reminders: Use phone alarms or calendar alerts for labs and imaging dates.
- Coordinate with your Hematology the medical specialty focused on blood disorders, including cancers like multiple myeloma team to ensure tests are ordered in the right order.
- Ask about trial eligibility: Many studies require recent baseline labs, and staying on schedule opens doors to novel therapies.
Even small logistics - planning transportation, confirming fasting status for labs, or arranging childcare - can make the difference between showing up or missing an appointment.

When to Call Your Doctor Outside of Routine Visits
Regular check‑ups are the safety net, but you shouldn’t wait for the next appointment if you notice warning signs. Contact your oncology team immediately if you experience:
- New or worsening bone pain, especially at night.
- Unexplained bruising or frequent infections.
- Sudden rise in calcium levels (symptoms: nausea, confusion).
- Rapid weight loss or persistent fatigue.
Early communication can trigger an unscheduled lab draw or imaging study, preventing a full-blown crisis.
Common Pitfalls and How to Avoid Them
Even motivated patients stumble. The most frequent errors include:
- Skipping blood work because they feel fine. Normal‑looking labs are the first sign that the disease is still under control.
- Missing imaging due to travel or cost concerns. Discuss insurance coverage or low‑dose options with your clinic.
- Ignoring mild side effects. Small changes in kidney function or blood counts can escalate if untreated.
Proactively addressing these issues with your care team keeps the treatment plan on track.
Long‑Term Outlook: Living Well with Multiple Myeloma
Thanks to modern therapies - proteasome inhibitors, immunomodulatory drugs, monoclonal antibodies, and CAR‑T cells - many patients now enjoy a decade or more of meaningful life after diagnosis. Regular check‑ups amplify those gains by ensuring each therapy stays effective and side effects are managed.
In practice, patients who schedule and attend regular check-ups for multiple myeloma report higher satisfaction scores and a stronger sense of control over their health journey.
Frequently Asked Questions
How often should a multiple myeloma patient get blood work?
During active treatment, labs are typically drawn every 2‑4 weeks. In the maintenance phase they are spaced to every 2‑3 months, and later years to every 3‑6 months, depending on risk factors.
Is a bone‑marrow biopsy required at every visit?
No. Biopsies are reserved for cases where blood tests or imaging suggest progression but are not definitive. Most follow‑ups rely on non‑invasive labs and scans.
What imaging modality is best for detecting early bone lesions?
Low‑dose whole‑body CT is fast and sensitive for cortical lesions, while MRI excels at spotting lesions in the spine and pelvis. Choice depends on prior findings and patient tolerance.
Can I skip a scheduled appointment if my last scans were clear?
Skipping appointments increases the chance of missing early signs of relapse. Even when scans are clear, routine labs and clinical assessment remain essential.
How do lifestyle factors affect the follow‑up schedule?
Good nutrition, regular exercise, and staying hydrated support bone health and kidney function, which can reduce the frequency of certain tests, but the core schedule usually stays the same.
Sticking to a disciplined follow‑up routine is one of the smartest moves a multiple myeloma patient can make. It turns uncertainty into data, giving you and your doctors the confidence to act swiftly, keep the disease in check, and live life on your terms.




Monika Bozkurt
October 19, 2025 AT 20:54In the realm of plasma‑cell dyscrasias, longitudinal monitoring constitutes a cornerstone of disease modifiability.
The implementation of scheduled surveillance protocols enables clinicians to capture subclinical perturbations in monoclonal protein kinetics.
By integrating serial serum free‑light‑chain quantification with imaging biomarkers, one can prognosticate relapse trajectories with heightened fidelity.
Consequently, patients who adhere to such regimens experience a quantifiable improvement in progression‑free survival.
From an existential standpoint, this disciplined vigilance transforms the oncologic narrative from one of passive endurance to active agency.
Penny Reeves
October 21, 2025 AT 00:51While the exposition attempts to masquerade as comprehensive, it scarcely scratches the surface of contemporary therapeutic stratagems.
One would expect a more rigorous dissection of minimal residual disease metrics rather than this pedestrian recapitulation.
Regrettably, the prose relegates itself to the realm of banal health‑blog fodder.
Sunil Yathakula
October 22, 2025 AT 04:48Hey fam, i totally get how overwhelming it can be to keep track of every lab and scan, but trust me, showing up even when you feel fine can save you from a nasty surprise later.
Just set up a simple calendar reminder, maybe a sticky note on the fridge, and you’ll be less likely to miss that crucial blood draw.
Remember, we’re all in this together, so don’t be afraid to lean on your support crew when the logistics get tricky.
Catherine Viola
October 23, 2025 AT 08:44It is incumbent upon the discerning practitioner to recognize that the ostensible emphasis on routine surveillance may serve as a veneer for pharmaceutical interests seeking to perpetuate chronic treatment cycles.
Indeed, the ubiquitous recommendation of perpetual imaging and biomarker assays aligns conspicuously with the revenue models of diagnostic enterprises.
Such alignment warrants a judicious appraisal of the underlying motivations dictating guideline formulations.
sravya rudraraju
October 24, 2025 AT 12:41Regular follow‑up appointments epitomize the disciplined framework required to navigate a disease as heterogeneous as multiple myeloma.
First and foremost, they afford clinicians the opportunity to recalibrate therapeutic intensity based on evolving laboratory indices such as serum M‑protein concentration and free‑light‑chain ratios.
Second, longitudinal imaging, whether via low‑dose whole‑body CT or MRI, unveils skeletal lesions that may otherwise evade detection until symptomatic fracture occurs.
Third, the incorporation of patient‑reported outcomes into each encounter cultivates a holistic understanding of quality‑of‑life dimensions that pure biometrics cannot capture.
Moreover, adherence to a structured surveillance schedule engenders a sense of empowerment in patients, fostering proactive health‑seeking behaviors.
Empirical data from the International Myeloma Working Group corroborate that systematic monitoring can reduce skeletal‑related events by up to twenty percent.
From a pharmacoeconomic perspective, early detection of relapse mitigates the need for more aggressive, cost‑intensive salvage regimens.
Clinicians should therefore view each visit not merely as a procedural checkpoint but as a strategic inflection point in the disease trajectory.
Patients are encouraged to maintain meticulous health journals, documenting pain scores, fatigue levels, and any new neurologic symptoms.
Such granularity enhances the physician’s ability to discern subtle trends that may presage biochemical progression.
In addition, synchronizing laboratory draws with imaging appointments optimizes resource utilization and minimizes patient inconvenience.
Healthcare teams must also prioritize clear communication regarding the rationale behind each test to bolster patient compliance.
When patients appreciate the purpose of periodic serum protein electrophoresis and immunofixation studies, they are less likely to dismiss them as superfluous.
Similarly, understanding the prognostic weight of a modest 0.5 g/dL rise in M‑protein can motivate timely interventions.
In sum, a rigorously adhered‑to follow‑up regimen synergizes clinical vigilance with patient agency, ultimately translating into extended overall survival.
Let us, therefore, champion a culture where regular check‑ups are celebrated as integral components of the therapeutic arsenal.
Ben Bathgate
October 25, 2025 AT 16:37Honestly, if you’re still scrolling through endless check‑up checklists, you’re just feeding the system’s paperwork obsession.
Most of those labs could be bundled, and half the imaging feels like a money‑grab for radiology departments.
Cut the fluff and focus on what actually moves the needle – a solid response to M‑protein spikes.
Ankitpgujjar Poswal
October 26, 2025 AT 19:34Listen up – skip a single appointment and you’re gambling with bone fractures and kidney failure.
Get your act together, set alarms, rope in a friend to drive you, and treat those labs like a lifeline.
Failure is not an option when your marrow health is on the line.
Bobby Marie
October 27, 2025 AT 23:30Skipping appointments is the fastest route to unmanaged relapse.
Christian Georg
October 29, 2025 AT 03:27Hey there! 😊 If you’re juggling appointments, try syncing all your blood work dates with a single calendar reminder and set a 24‑hour pre‑alert.
This way you won’t miss the fasting window for labs, and you can batch imaging on the same day to save time and travel costs.
Consistent reminders are a simple hack that many patients find lifesaving.
Christopher Burczyk
October 30, 2025 AT 07:24The stratification of surveillance intervals, as delineated by NCCN, is predicated upon risk‑adapted modeling that incorporates cytogenetic aberrations, depth of response, and prior therapy intensity.
Consequently, high‑risk cohorts necessitate bi‑monthly serologic assessments, whereas low‑risk patients may safely transition to quarterly evaluations after sustained remission.
Adherence to these evidence‑based schedules optimizes both clinical outcomes and resource allocation.
Nicole Boyle
October 31, 2025 AT 11:20From a pragmatic viewpoint, the longitudinal assessment of serum free‑light‑chain kinetics functions as a surrogate for clonal burden, while serial low‑dose CT furnishes spatial resolution of osseous involvement.
In practice, patients who integrate these biomarkers into their routine monitoring exhibit a modest yet statistically significant extension in progression‑free intervals.
It’s a classic example of how quantitative analytics dovetail with radiologic surveillance to refine therapeutic decision‑making.
Caroline Keller
November 1, 2025 AT 15:17We are all supposed to be vigilant guardians of our own bodies but some people act like they are immune to the consequences of negligence and it is absolutely disgraceful to watch such reckless complacency gnaw away at the very fabric of a community that should be united in fighting this relentless disease.
dennis turcios
November 2, 2025 AT 19:13The article does a decent job of outlining the standard follow‑up components, yet it glosses over the socioeconomic barriers that many patients face when trying to adhere to such intensive schedules.
A more nuanced discussion of insurance hurdles and transportation challenges would provide a fuller picture.
As it stands, the piece feels somewhat detached from the lived reality of many myeloma patients.
Felix Chan
November 3, 2025 AT 23:10Keep that positive vibe going and treat each check‑up like a win – every lab result is a checkpoint on your journey, not a dreaded chore.
Thokchom Imosana
November 5, 2025 AT 03:06One must remain circumspect about the ostensibly altruistic veneer of incessant surveillance protocols, for beneath the polished recommendations lies a labyrinthine agenda orchestrated by pharmaceutical conglomerates and diagnostic conglomerates eager to perpetuate a perpetual treatment loop.
The relentless push for quarterly imaging, ostensibly justified by early detection imperatives, in fact fuels a lucrative market for high‑resolution scanners, while the mandated bi‑monthly serum free‑light‑chain assays secure a steady stream of consumable revenue for laboratory enterprises.
Such symbiotic interactions between industry stakeholders and guideline committees raise legitimate concerns regarding the true motivations governing these ostensibly evidence‑based directives.
Patients, therefore, would do well to interrogate the underlying economic incentives and seek second opinions that prioritize clinical necessity over commercial expediency.